Aph 162 Biological Physics LaboratoryDavid Van Valen Michael Amori |
|||||||||||||||||||||||||||||||||||||||||||||||
Sizes Rates DNA Science |
The Size of Things
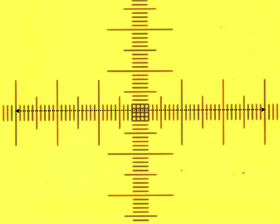
The goal of this segment of the course was to get a feel for the size of biological organisms. The first step was to calibrate our microscopes to obtain a conversion from voxels to an actual length. To do this, we viewed essentially looked at a ruler with different objectives and measured known lengths in terms of voxels.
Figure 1: Calibrating the microscope This calibration gave us the conversion
With these conversion factors, we then examined three organisms: Stentor, E. Coli, and C. Elegans. Stentor
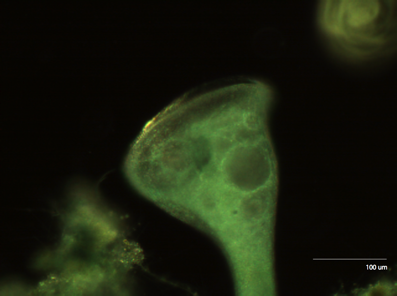
The first organism we looked at was Stentor, one of the largest unicellular organisms available.
Figure 2: Stentor at 10x Upon closer inspection, we can see the collections of cilia (cirri) that stentor uses to generate the vortices that propel food into its mouth.
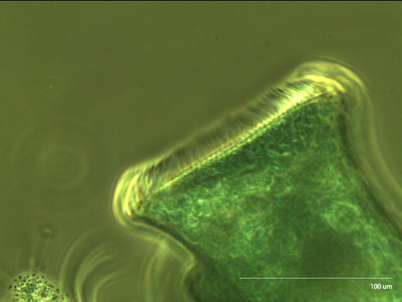
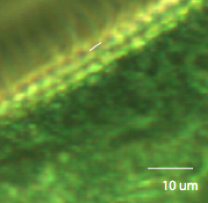
Figure 3: Stentor at 20x Zooming in, we can estimate the spacing between cirri to be about 12.2 voxels, or 3.146 um.
Figure 4: Estimating the distance between
cirri E. Coli
The second species we examined was E. Coli, which has been the benchmark organism for this course. E. Coli has a remarkable ability to utilize plasmids to alter its pathogenicity. The bacteria we observed were, of course, devoid of any such modifications.
Figure 5: E. Coli on a glass slide.
Photograph taken at 100x E. Coli is too small to be properly visualized at less than 100x. In order to use the 100x objective, we had to place a drop of oil between the objective and our sample. The oil has the same refractive index as the glass coverslip and the objective, and serves to remove coverslip-air and air-objective interfaces to allow for higher resolution. The images we captured appear to have been taken just after a cell division. The most interesting measurement we can make is the size of E. Coli. One cell is approximately 47.67 voxels, or 2.46 um. C. Elegans
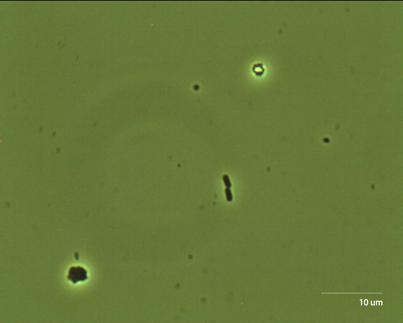
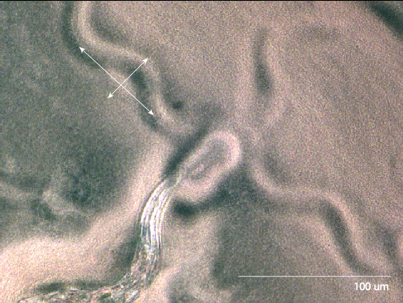
The third species we observed was C. Elegans. http://snowdome.caltech.edu/aph162/David and Michael/worm2.avi By looking at the indentation the worm makes in the media, we can measure its amplitude and its wavelength. |
||||||||||||||||||||||||||||||||||||||||||||||
|
Sperm |
Velocity (um/s) |
|
1 |
3.5862 |
|
2 |
3.4445 |
|
3 |
3.8090 |
|
4 |
4.0589 |
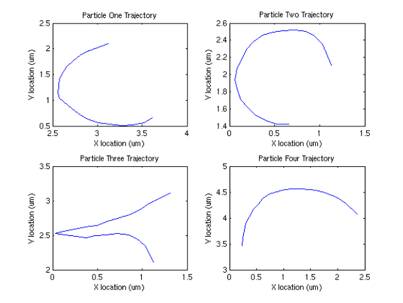
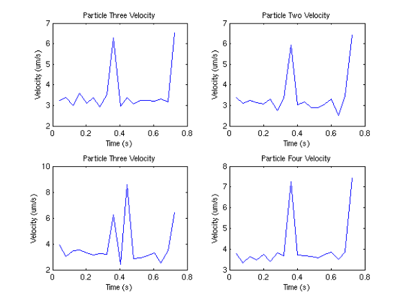
Interestingly, we can also compute the correlation between velocities of different sperm.
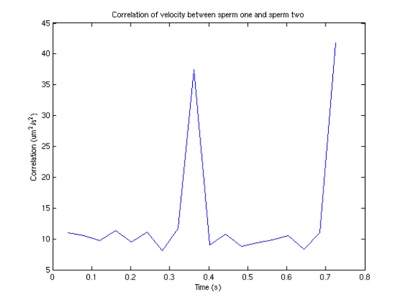
Figure 9: Velocity correlation between
sperm 1 and sperm 2
From this graph, we can see that surprisingly (although not surprising if one looks at the data) that the velocities are correlated, and there appears to be a periodicity of about .4 seconds in the velocity. One possible explanation (courtesy of Tristan Ursell) is that the sperm are not free in solution. It is possible that the flagella of the sperm are tethered to the bottom of the glass slide. This would account for the circular motion we see, which would also explain the correlation in velocities.
To calculate the rate of ATP consumption, we will assume that the viscous forces that act on the sperm can be modeled by StokeÕs law,
where ![]() is the viscosity, r is the radius of
the sperm, and v is itÕs velocity. To keep a constant velocity, ATP must be
converted to ADP to produce enough energy to oppose the work being done by
the viscous fource. So the energy per second needed is
is the viscosity, r is the radius of
the sperm, and v is itÕs velocity. To keep a constant velocity, ATP must be
converted to ADP to produce enough energy to oppose the work being done by
the viscous fource. So the energy per second needed is
Using the values,
|
Quantity |
Value |
|
|
8.90*10-4 Ns/m2 |
|
R |
5.79 um |
we see that the energy expended per second is
|
Sperm |
Energy/second (J/s) |
|
1 |
1.249*10-18 |
|
2 |
1.153*10-18 |
|
3 |
1.409*10-18 |
|
4 |
1.600*10-18 |
The energy generated from the hydrolysis of ATP is 7.3 kcal/mol, which is 5.07*10-20 J/molecule. Using this value, the ATP consumed by the sperm per second is
|
Sperm |
ATP/second (molecules/s) |
|
1 |
24.6 |
|
2 |
22.7 |
|
3 |
27.8 |
|
4 |
31.6 |
So an average of 26.7 ATP/second is consumed to propel the sperm through the aqueous media.
DNA Science
The third segment of the course focused on DNA science and the protocols behind manipulating genomes. The goal for this part was simple: we wanted to create bacterial cell that expressed lacZ with a tetracycline inducible promoter. The pieces we started with were
- A vector with an gene for kanamycin resistance and GFP
- DNA from E Coli and plasmids that contained lac Z
The protocol was essentially cut and paste. We first used restriction enzymes to cut our vector, then ran it through a polyacrilimide gel to purify the portion that had the antibiotic resistance. We then PCR amplified the lacZ region of our DNA using primers that would give us the appropriate sticky ends we needed for ligation. We then combined out cut vector with our lacZ insert with DNA ligase to obtain a new plasmid. We then transformed bacterial cells with our plasmid using electroporation, and plated them on agarose gels that contain IPTG and kanamycin. Cells that were successfully transfected with the plasmid will express beta-galactosidase, which will cleave IPTG, causing a blue mark to appear on the gel. The results from this portion of the lab are still pending.
References
- Wikipedia
- Alberts, Molecular Biology of the Cell