We studied two key
biological polymers: actin and microtubules (MT).
This was done using both bulk and single molecule techniques.
Microtubule Polymerization
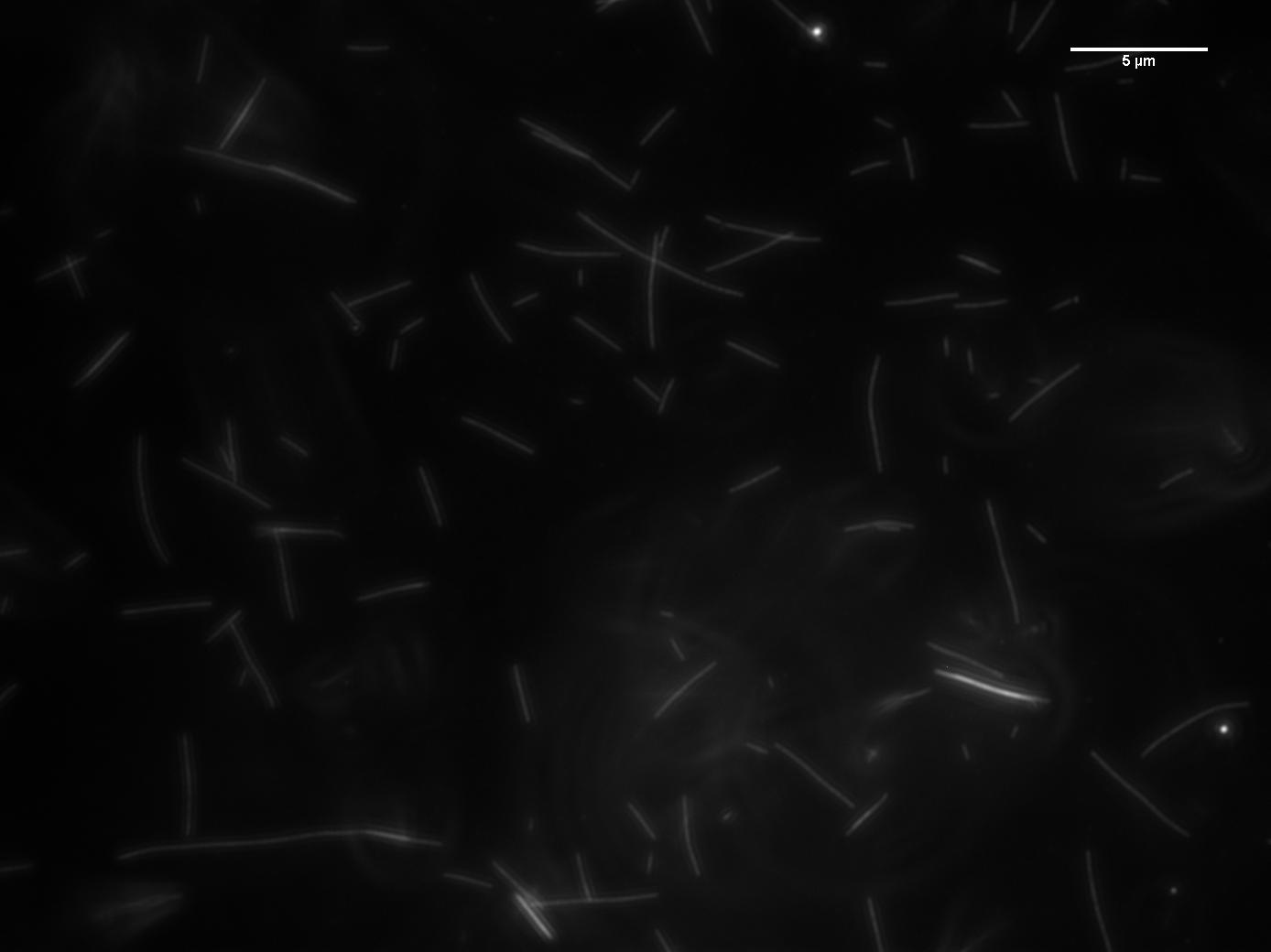 |
Rhodamine-labelled
tubulin was polymerized at different monomer concentrations.
The current state of polymerization can be preserved
in a two step process: the molecule Taxol is added to
prevent depolymerization, and the solution is quickly
diluted reducing the tubulin monomer concentration and
hence preventing further polymerization.
In this way snapshots of the reaction can be obtained
under the microscope.
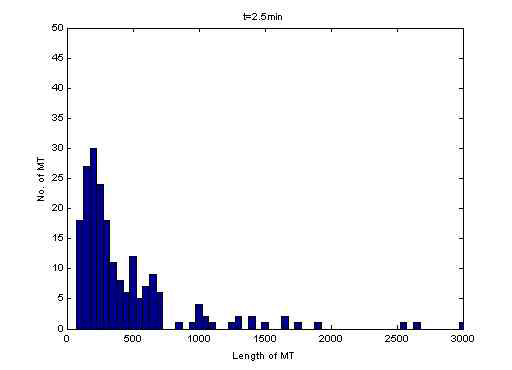
The students wrote
Matlab code to analyze the distribution of filament lengths.
By stopping the reaction at different time points information
about the
kinetics of polymerization can be obtained. |
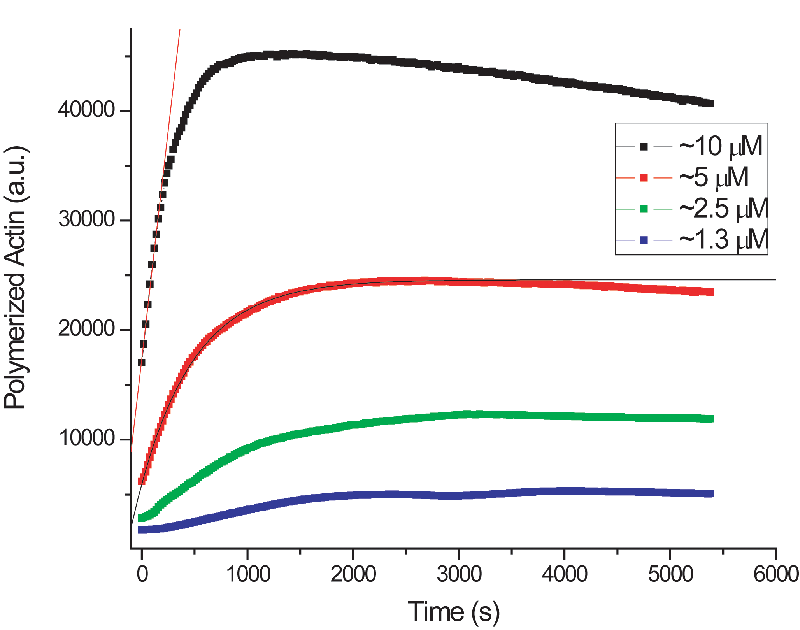
Actin Polymerization
| Similar to the
beta-galactosidase assay (The Rate of
Things), students
used kinetic spectrophotmetry to watch the bulk polymerization
of actin filaments and deduce the monomer association rate.
Pyrene-labelled actin becomes fluorescent only upon polymerization;
this
gives a direct readout of the amount of actin in the filamentous
form. The polymerization rate can be studied at
different concentrations of monomer or in the presence
of protein factors like Arp2/3. As with beta-gal, the polymerization
is a linear kinetic process. |
 |

